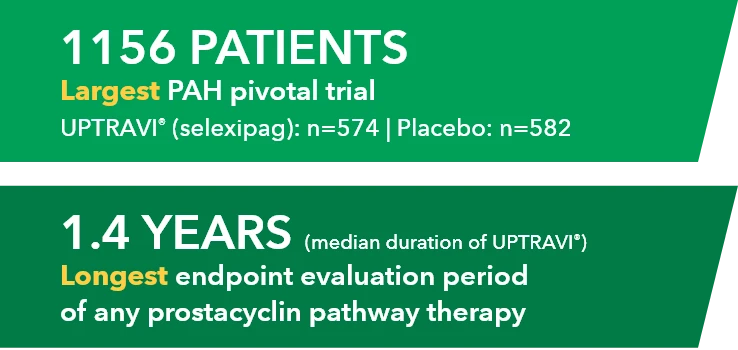
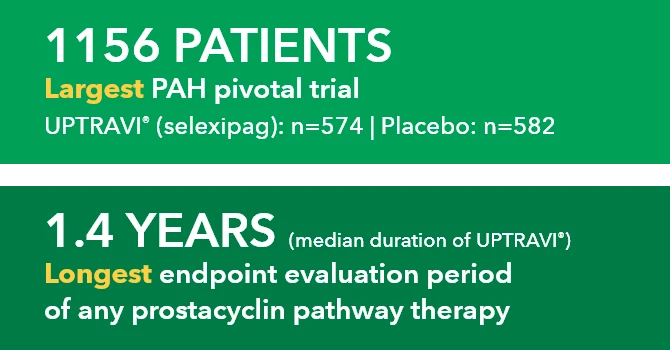
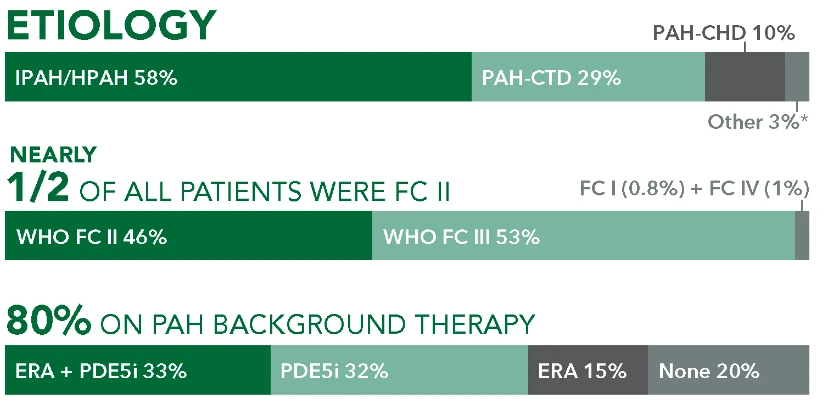
GRIPHON: The Largest Pivotal Trial Conducted in PAH (N=1156)1-3
The safety and efficacy of UPTRAVI® was demonstrated in a multicenter, double-blind, placebo-controlled, parallel-group, event-driven study in patients with symptomatic PAH (>98% WH FC II or III). The primary endpoint was the time to first disease progression event.* Treatment with UPTRAVI® resulted in a 40% risk reduction† (99% CI: 22% to 54%; P<0.0001; HR 0.60) in disease progression compared with placebo (27% [155/574] vs 41.6% [242/582], respectively). Adverse reactions occurring more frequently (≥5%) on UPTRAVI® compared with placebo are headache, diarrhea, jaw pain, nausea, myalgia, vomiting, pain in extremity, and flushing.1,4
GRIPHON OPEN-LABEL EXTENSION:
10 Years of Data: The Longest Follow-up Period of Any PAH Therapy5*
Estimates of survival for patients across GRIPHON and open-label extension studies (95% CI)6†

UPTRAVI® (selexipag) demonstrated consistent, long-term safety across GRIPHON (N=1156) and open-label extension1,5
These data are from long-term follow-up and an open-label extension study. These uncontrolled observations do not allow comparison with a control group not given UPTRAVI® and cannot be used to determine the long-term effect of UPTRAVI® on mortality.
At Year 10, 408 patients had been censored. Participant is said to be censored when information on time to event is not available due to loss to follow-up or non-occurrence of outcome event before the trial end.6,7
A long-term follow-up was conducted in patients who were treated with UPTRAVI® in the pivotal trial and OLE (N=574).6