Pivotal trial overall population: Probability of hospitalization event1*

Percentage of patients hospitalized for PAH as a first event occurring as part of the composite endpoint1

13.6%
p>(n=78)
UPTRAVI®
18.7%
v
(n=109)



Clicking CONTINUE below will take you to the selected site, the content for which Johnson & Johnson is not responsible and to which this Privacy Policy does not apply. We encourage you to read the Privacy Policy of every online service you visit.



The following form is intended for use with patients who are eligible for VA benefits only.

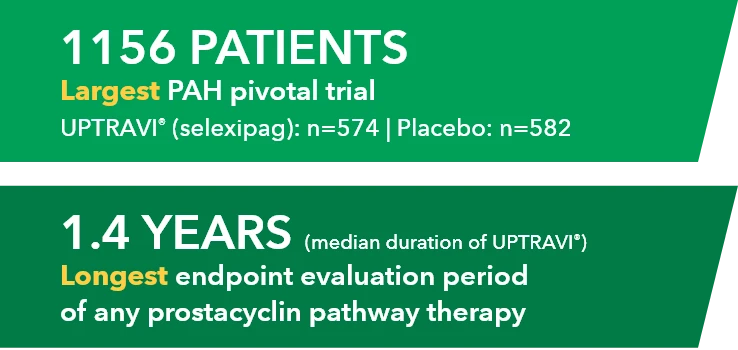
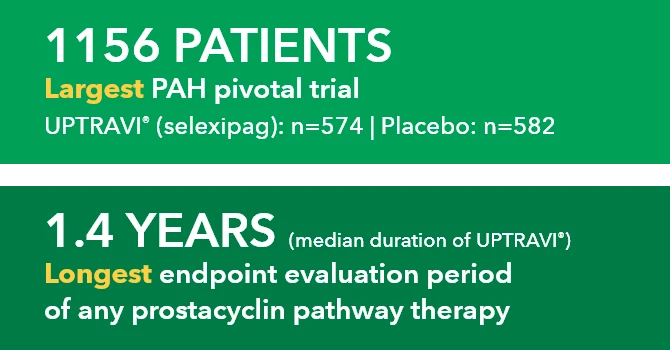
1156 PATIENTS Largest PAH pivotal trial UPTRAVI" (selexipag): n-574 | Placebo: n-582
1.4 YEARS (median duration of UPTRAVI*) Longest endpoint evaluation period of any prostacyclin pathway therapy
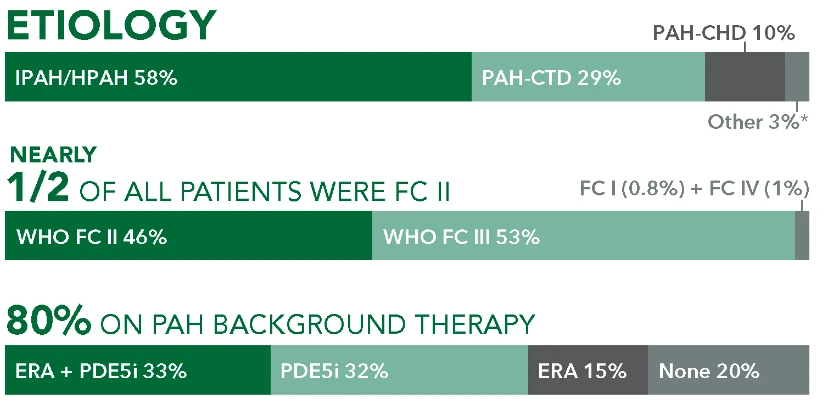
ETIOLOGY
IPAH/HPAH 58%
PAH-CHD 10%
PAH-CTD 29%
Other 3%*
NEARLY 1/2 OF ALL PATIENTS WERE FC II
WHO FC II 46%
FC I (0.8%) + FC IV (1%)
WHO FC III 53%
80% ON PAH BACKGROUND THERAPY
ERA + PDE5i33%
PDE5i 32%
ERA 15%
None 20%
This article includes information that has not been approved by the Food and Drug Administration for UPTRAVI® (selexipag). Please see full Prescribing Information available on this website. Authors[s] of this article have received remuneration from Actelion Pharmaceuticals US, Inc., or its affiliates.