- Multicenter, long-term, double-blind, placebo-controlled, parallel-group, event-driven, phase 3 trial1,4
Study Design1-3
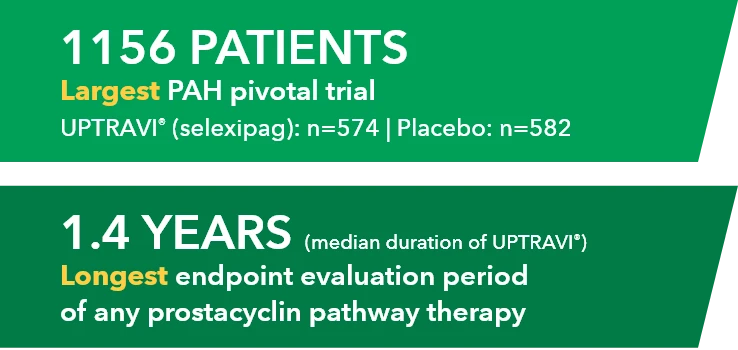
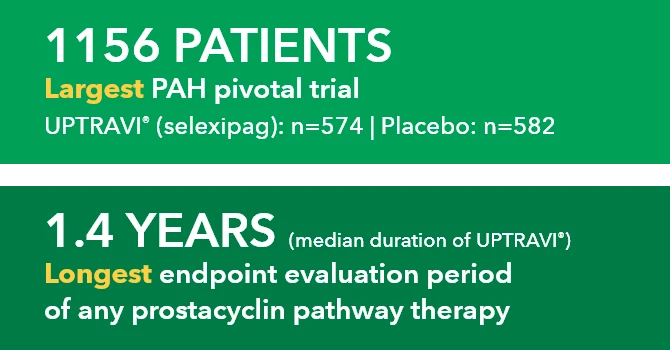
1156 PATIENTS Largest PAH pivotal trial UPTRAVI" (selexipag): n-574 | Placebo: n-582
1.4 YEARS (median duration of UPTRAVI*) Longest endpoint evaluation period of any prostacyclin pathway therapy
Baseline Patient Characteristics1,5
- Mean age: 48 years
- Female: 80%
- Median time from PAH diagnosis in patients taking UPTRAVI®: 0.9 years
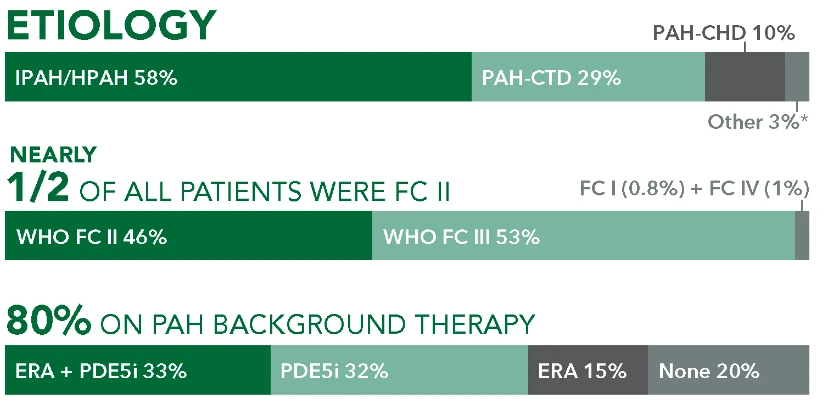
ETIOLOGY
IPAH/HPAH 58%
PAH-CHD 10%
PAH-CTD 29%
Other 3%*
NEARLY 1/2 OF ALL PATIENTS WERE FC II
WHO FC II 46%
FC I (0.8%) + FC IV (1%)
WHO FC III 53%
80% ON PAH BACKGROUND THERAPY
ERA + PDE5i33%
PDE5i 32%
ERA 15%
None 20%
This article includes information that has not been approved by the Food and Drug Administration for UPTRAVI® (selexipag). Please see full Prescribing Information available on this website. Authors[s] of this article have received remuneration from Actelion Pharmaceuticals US, Inc., or its affiliates.



