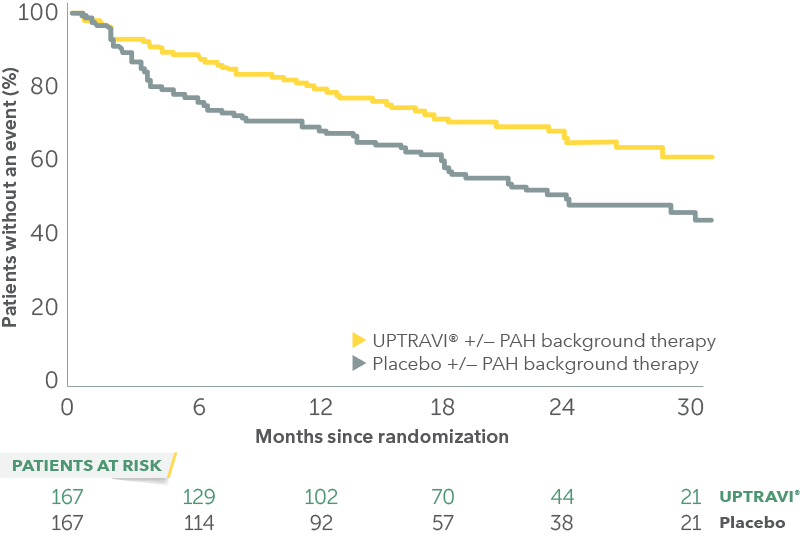
In an exploratory post hoc subgroup analysis, UPTRAVI® was associated with a: 41% RELATIVE RISK REDUCTION OF DISEASE PROGRESSION IN PAH-CTD PATIENTS TREATED WITH UPTRAVI® VS PLACEBO1*
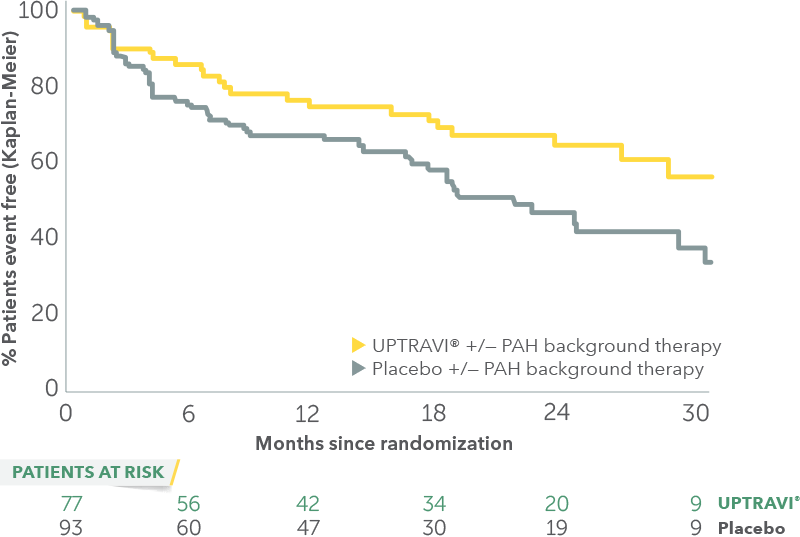
Time to first disease progression event in patients with PAH-CTD

Baseline patient characteristics
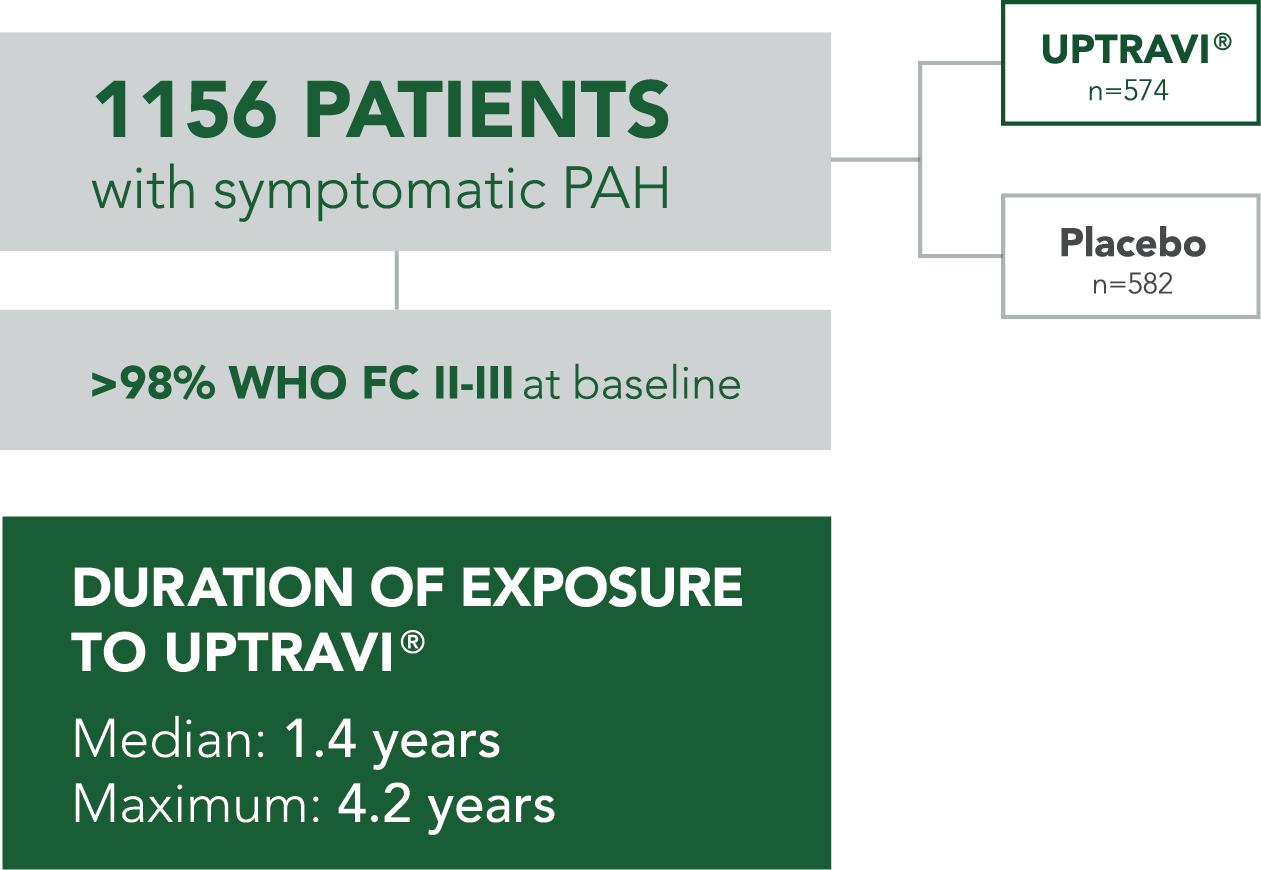
- 29% (n=334) of all patients in GRIPHON had PAH-CTD
- PAH-CTD subtypes: PAH-SSc (51%, n=170), PAH-SLE (24.5%, n=82), PAH-MCTD/CTD-Other (24.5%, n=82)†
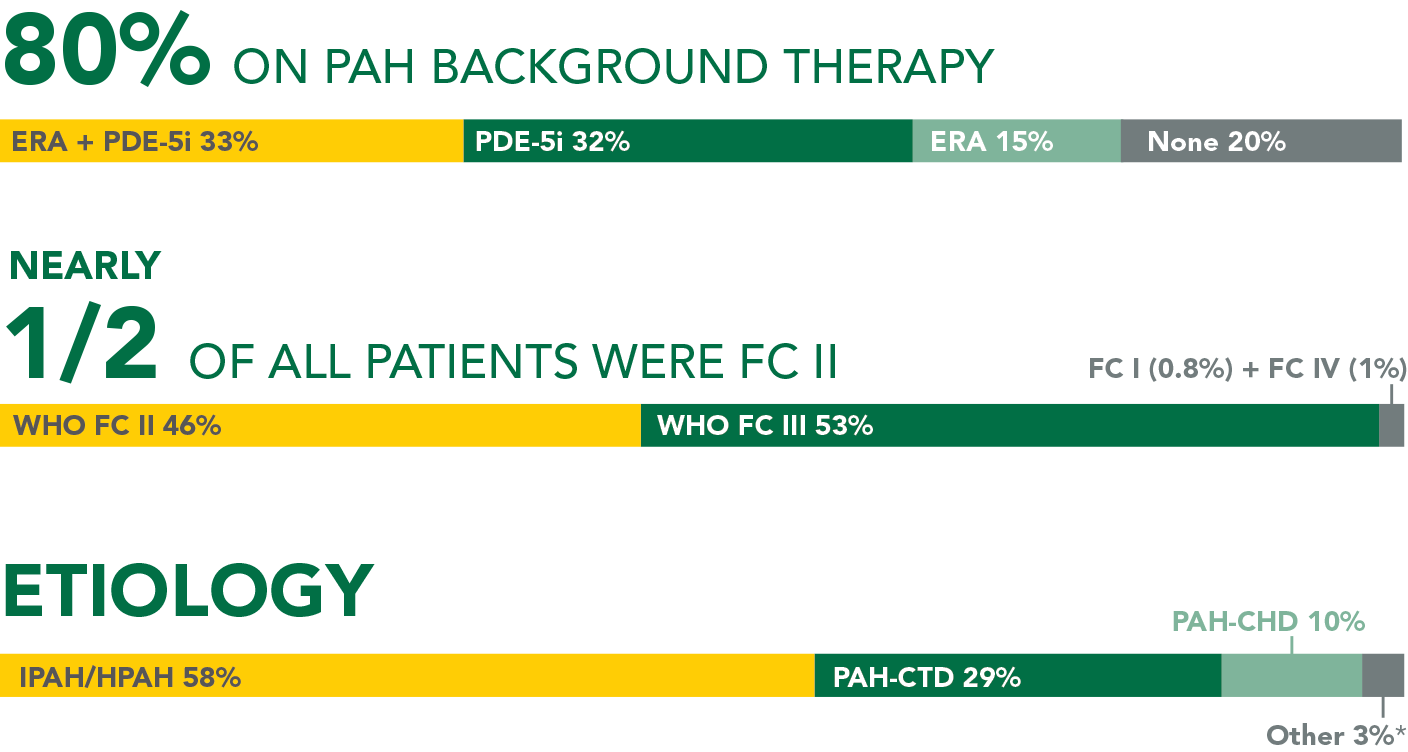
- 46% of patients in the PAH-CTD subgroup were FC II and 53% were FC III
- 28% were receiving PDE-5i monotherapy, 23% were not receiving PAH background therapy, 20% were receiving ERA monotherapy, and 29% were receiving an ERA + PDE-5i at baseline
- Time from diagnosis: UPTRAVI® (1.6 years), placebo (1.7 years)
- Average age: UPTRAVI® (52 years), placebo (53 years)
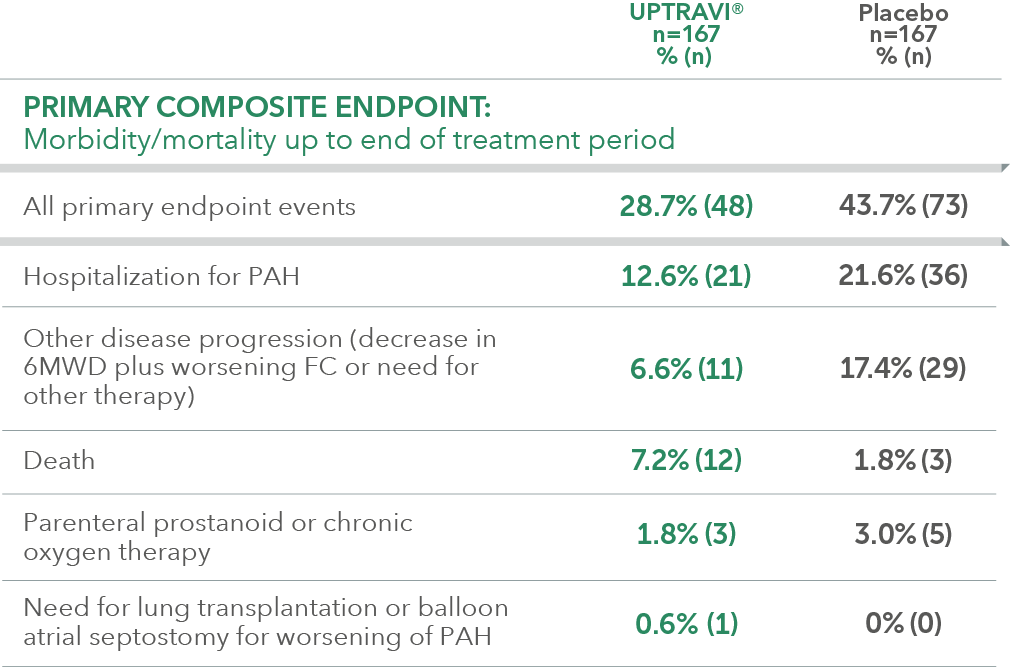
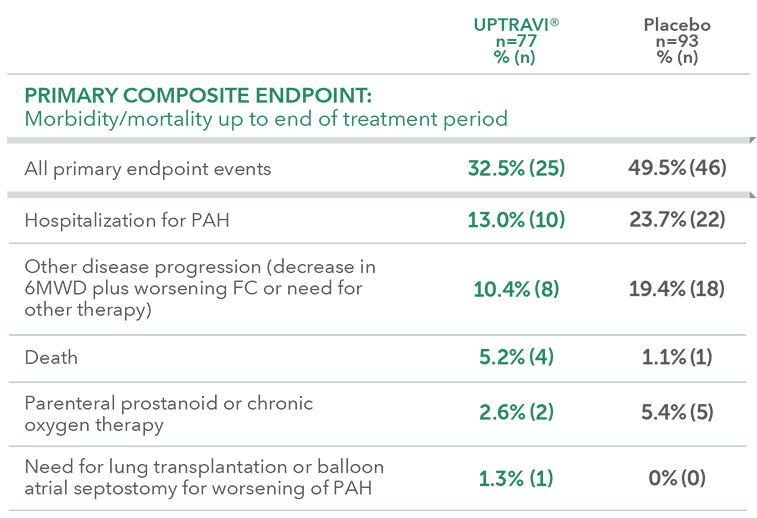
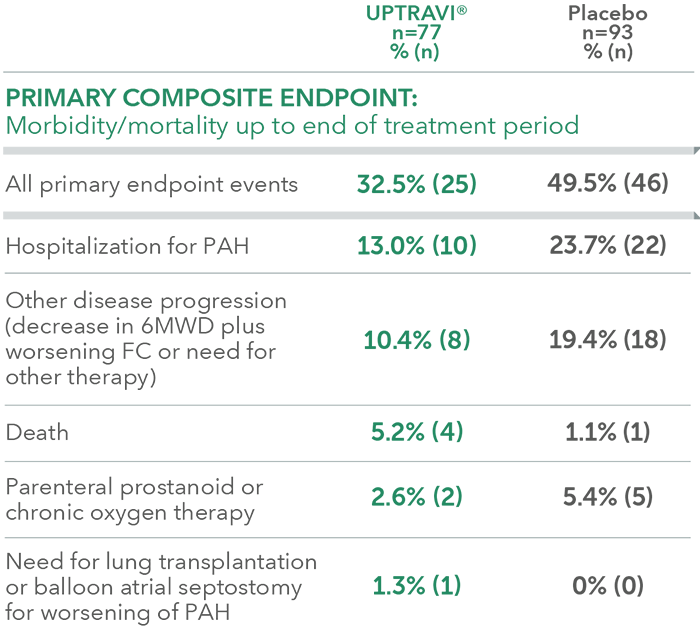
Summary of primary endpoint events in patients with PAH-CTD
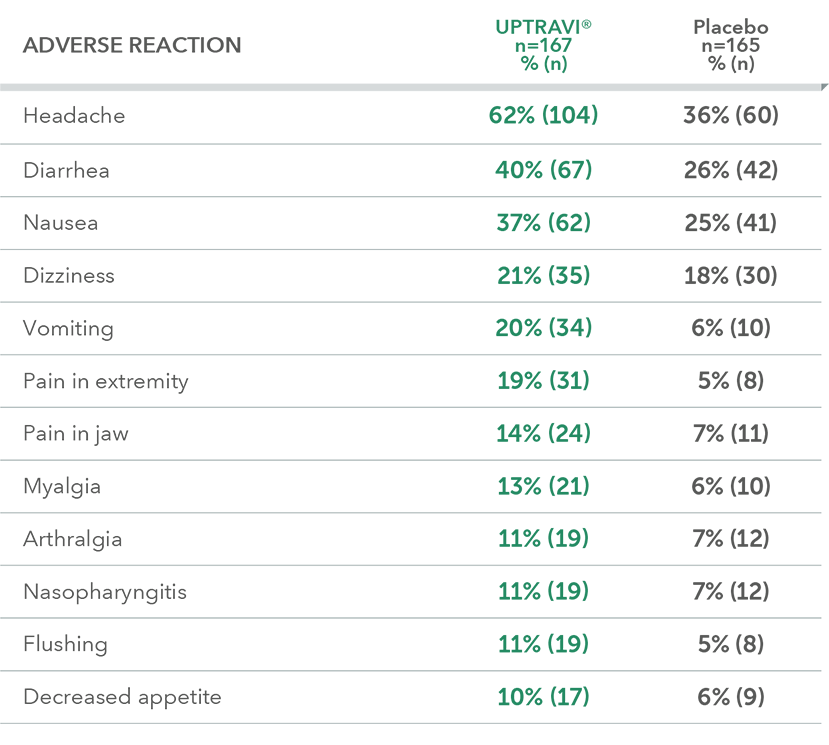
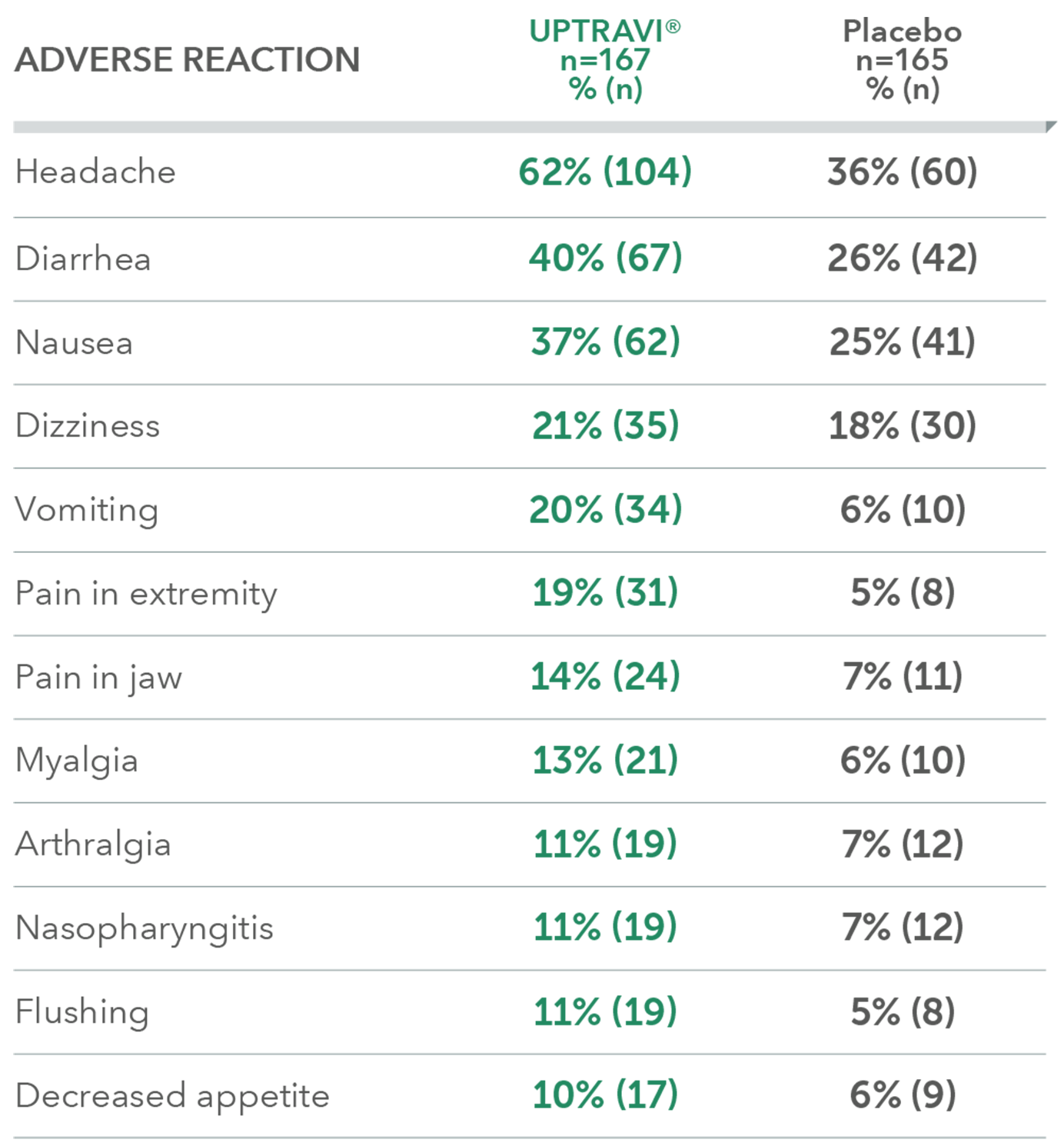
Adverse reactions in the PAH-CTD subpopulation occurring more frequently with UPTRAVI® compared with placebo by ≥3%
PAH-CTD was a prespecified subgroup for evaluation of the primary endpoint; however, the more detailed analyses described here are post hoc. Sample size should be considered and results should be interpreted with caution.
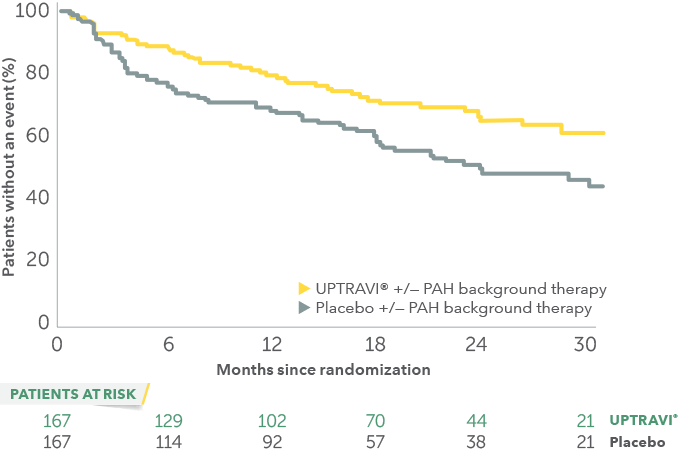
In an exploratory post hoc subgroup analysis, UPTRAVI® was associated with a: 44% RELATIVE RISK REDUCTION OF DISEASE PROGRESSION IN PAH-SSc PATIENTS TREATED WITH UPTRAVI® VS PLACEBO1*
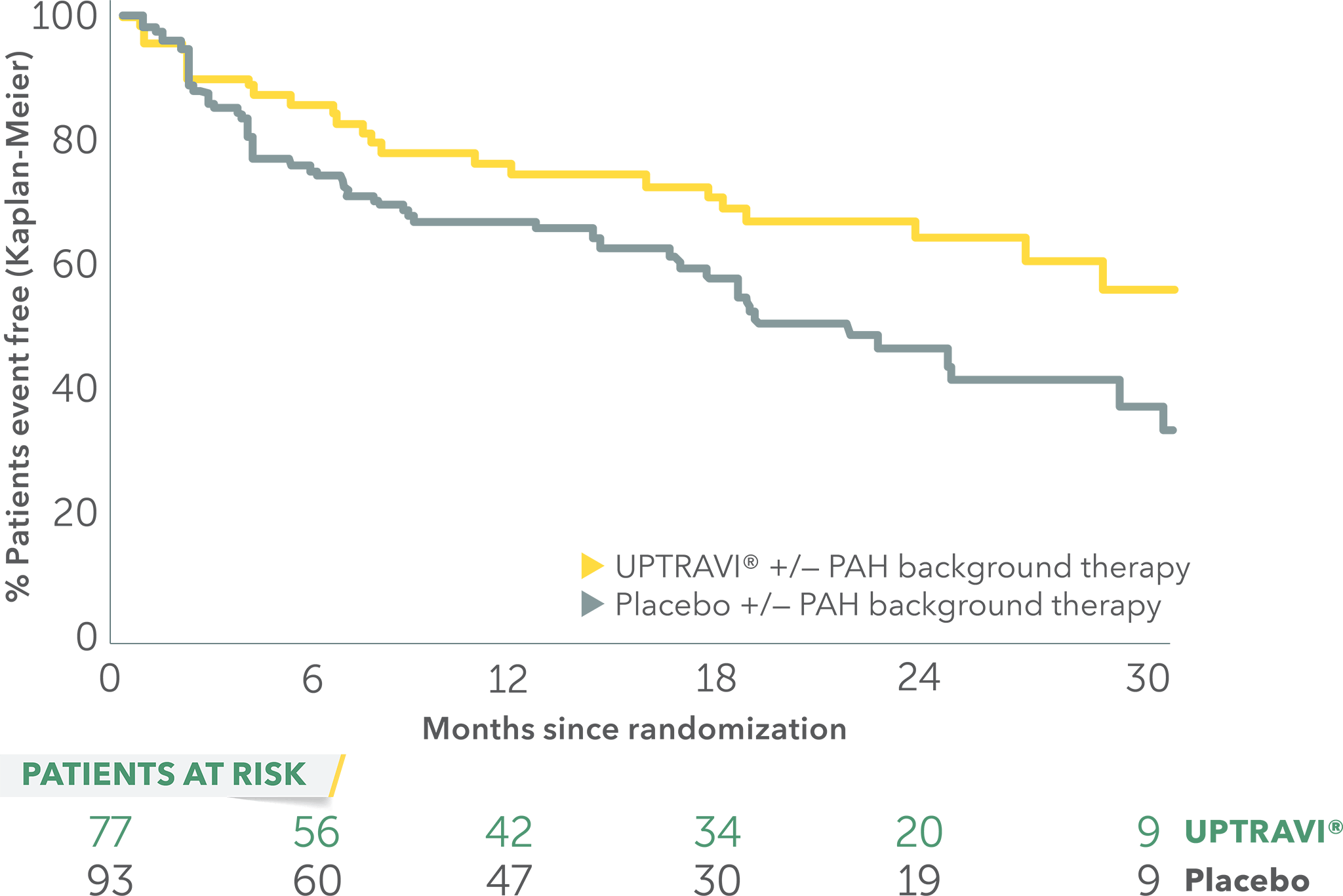
Time to first disease progression event in patients with PAH-SSc

Baseline patient characteristics
Of the 334 patients with PAH-CTD in GRIPHON, 51% (n=170) had PAH-SSc.
- 34% FC II, 65% FC III, and 2% FC I or FC IV
- 78% of patients were receiving PAH background therapy (36% receiving an ERA + PDE-5i, 23% receiving a PDE-5i only, and 18% receiving an ERA only)
- Time from diagnosis: UPTRAVI® (1.5 years), placebo (1.6 years)
- Average age: UPTRAVI® (59 years), placebo (61 years)
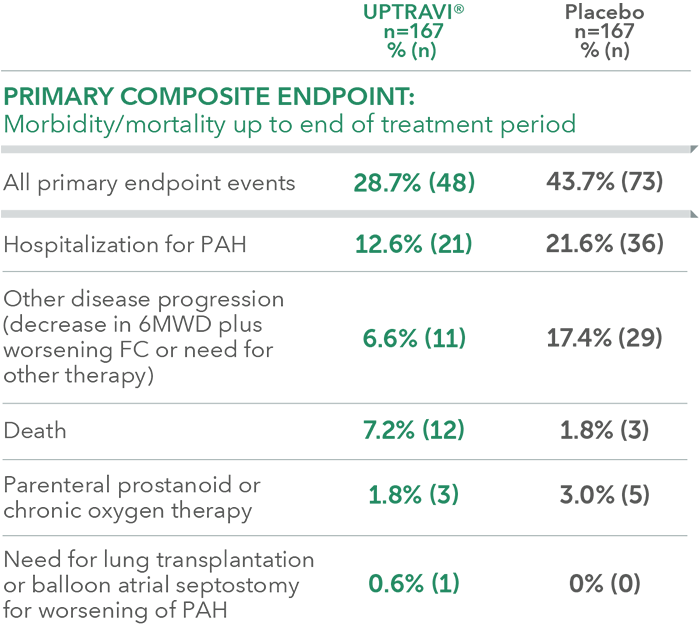
Summary of primary endpoint events in patients with PAH-SSc
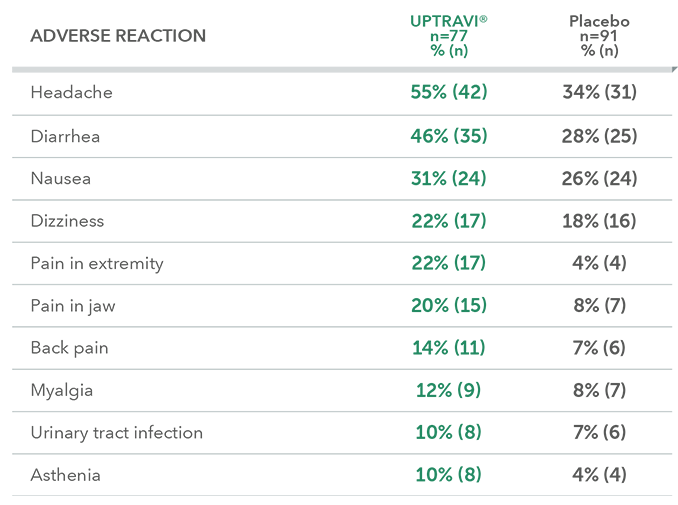
Adverse reactions in the PAH-SSc subpopulation occurring more frequently with UPTRAVI® compared with placebo by ≥3%
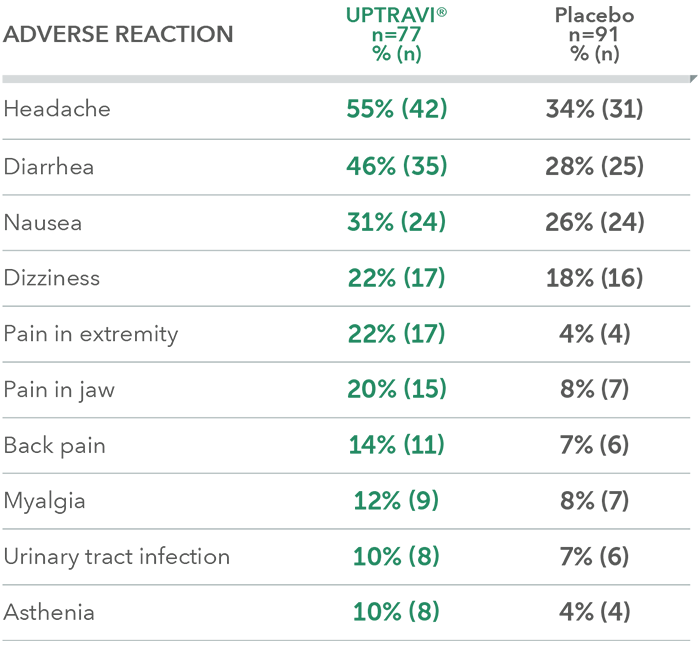
This subgroup analysis was post hoc and exploratory in nature. Sample size should be considered and results should be interpreted with caution.