Estimates of survival for patients across GRIPHON and open-label extension studies (95% CI)2†

92.0% (89.4, 94.0) (82.0, 88.0)Median follow-up time 85.3% 71.2% (66.5, 75.3) 63.3% (57.8, 68.3) 56.9% (50.3,62.9) (50.3, 62.9) 56.9% Year 1
PATIENTS AT RISK
501
Year 2
Year 3
Year 4
Yoar 5
Year 6
Yoar 7
Yoar 8
Yoar 9
Year 10
39 305 235 177 13 119 89 44 10
Patients taking UPTRAVI® had a 56.9% estimate of survival at Year 10
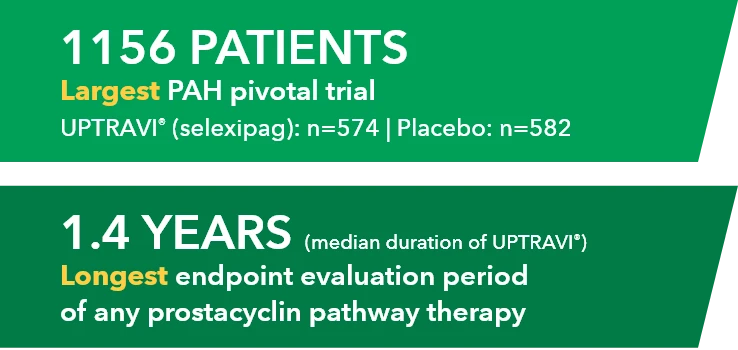
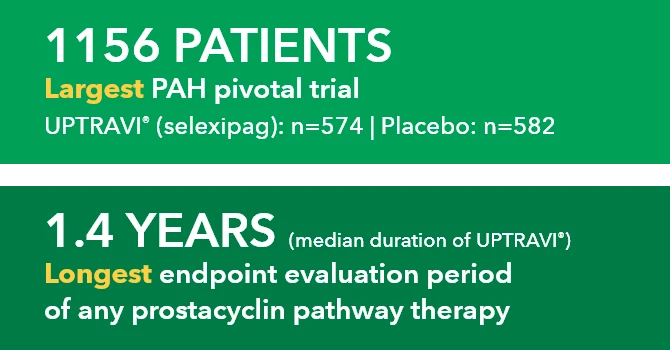
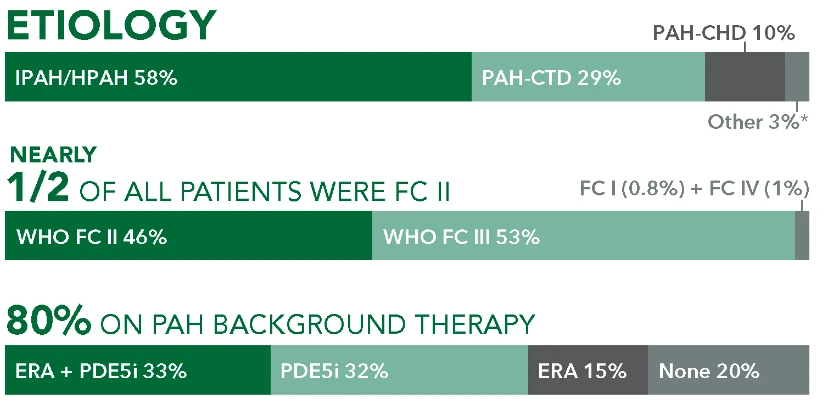
UPTRAVI® (selexipag) demonstrated consistent, long-term safety across GRIPHON (N=1156) and open-label extension1,3
These data are from long-term follow-up and an open-label extension study. These uncontrolled observations do not allow comparison with a control group not given UPTRAVI® and cannot be used to determine the long-term effect of UPTRAVI® on mortality.
At Year 10, 408 patients had been censored. Participant is said to be censored when information on time to event is not available due to loss to follow-up or non-occurrence of outcome event before the trial end.2,4
A long-term follow-up was conducted in patients who were treated with UPTRAVI® in the pivotal trial and OLE (N=574).2