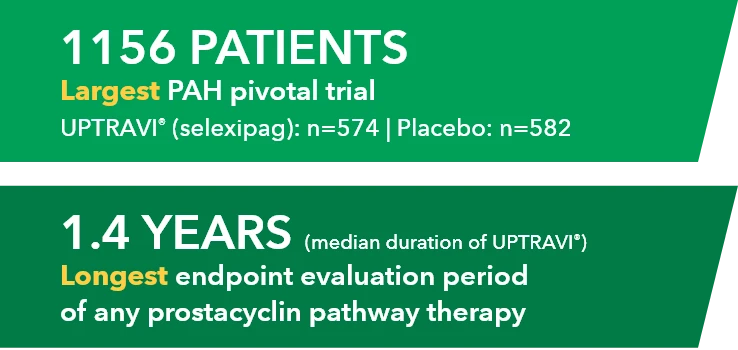
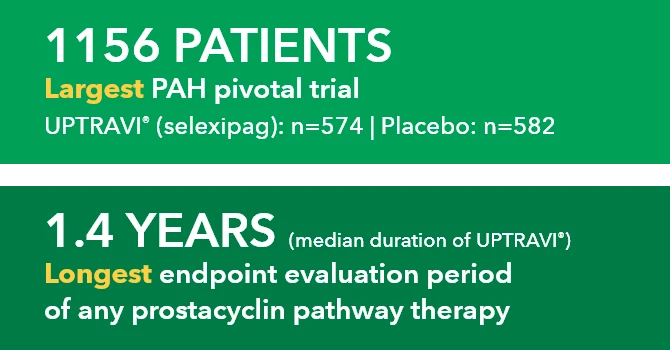
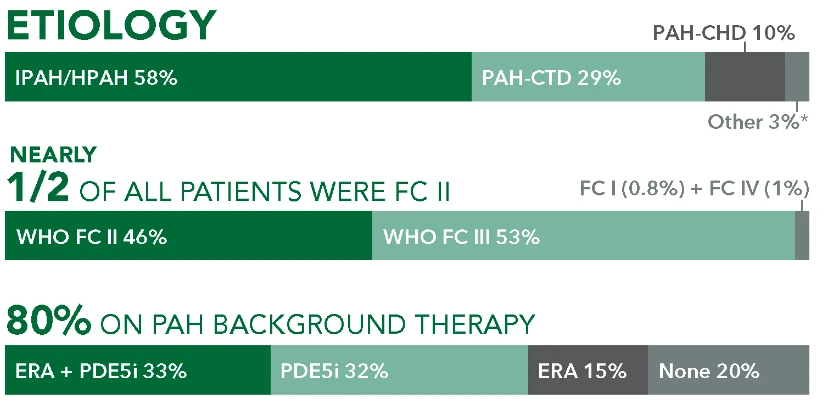
When you decide to start your patient on treatment with UPTRAVI®, it’s important to set goals and expectations with them.
Before starting UPTRAVI®, remind them of the following:
- Anticipate a call from your specialty pharmacy to alert you of your next shipment of UPTRAVI®
- Do not change your dose or stop taking UPTRAVI® unless your doctor tells you to do so1
- Understand expectations for potential side effects. Follow up with your doctor if you have side effects that you cannot tolerate1
- Try to take UPTRAVI® with food. This may help you tolerate the dose better if you are having side effects with UPTRAVI®1
- After the dose adjustment phase, you will have found your personal maintenance dose of UPTRAVI®. This will be the dose you continue to take on a regular basis, unless your doctor tells you otherwise1
UPTRAVI® Dose Adjustment Phase—Helps Meet Individual Patient Needs1
Your patient's recommended starting dose is 200 mcg twice daily. UPTRAVI® dosing is unique to each patient based on how their body responds and adjusts to treatment. The dose adjustment phase is how you find your patient's personal maintenance dose.

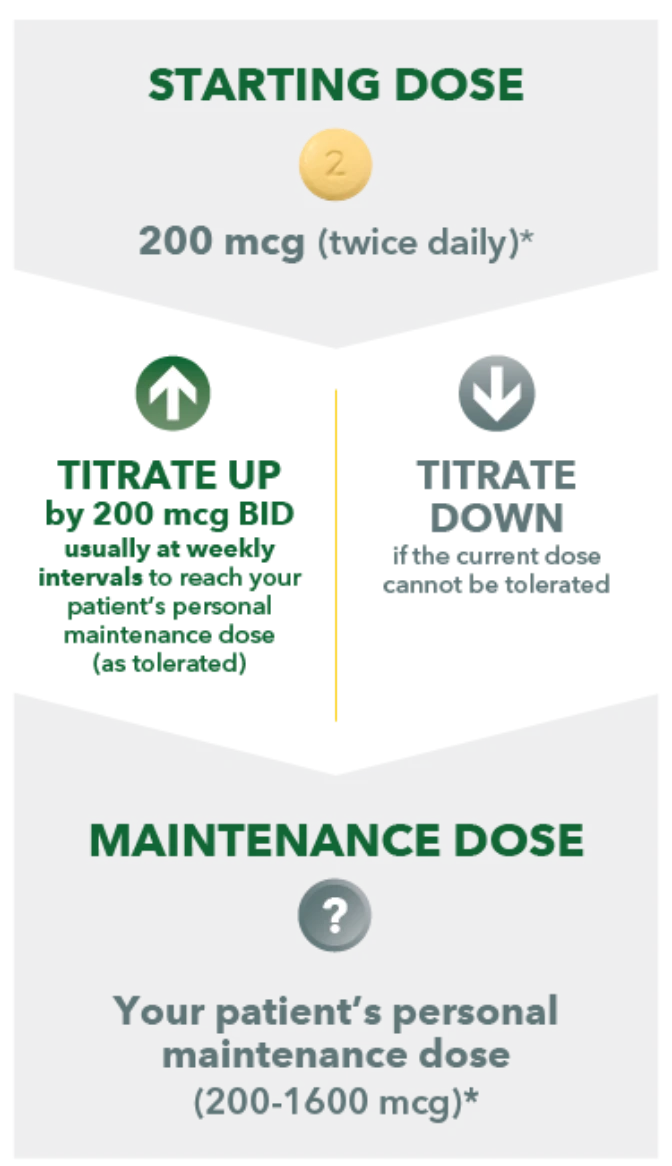
STARTING DOSE 2 200 mcg (twice daily)* TITRATE UP by 200 mcg BID usually at weekly intervals to reach your patient's personal maintenance dose (as tolerated) TITRATE DOWN if the current dose cannot be tolerated MAINTENANCE DOSE
?
Your patient's personal
maintenance dose
(200-1600 mcg)*
*Once daily for patients with moderate hepatic impairment (Child-Pugh class B) and co-administration with moderate CYP2C8 inhibitors (eg, clopidogrel, deferasirox, and teriflunomide). Increase in increments of 200 mcg once daily at weekly intervals.
- If a dose of UPTRAVI® is missed, patients should take the missed dose as soon as possible unless the next dose is within the next 6 hours
- If treatment is missed for 3 days or more, restart UPTRAVI® at a lower dose and then retitrate
After the dose adjustment phase1
Once each patient reaches their personal dose (maintenance), a single-tablet equivalent is prescribed BID. There are 8 different single-tablet strengths of UPTRAVI®, each a unique color.
UPTRAVI® OFFERS A RANGE OF DOSE STRENGTHS TO ACCOMMODATE EACH PATIENT’s personal MAINTENANCE dose









UPTRAVI® Offers Oral Administration With Similar Efficacy at Every Dose Group1
Time to first disease progression event in GRIPHON by dose group3
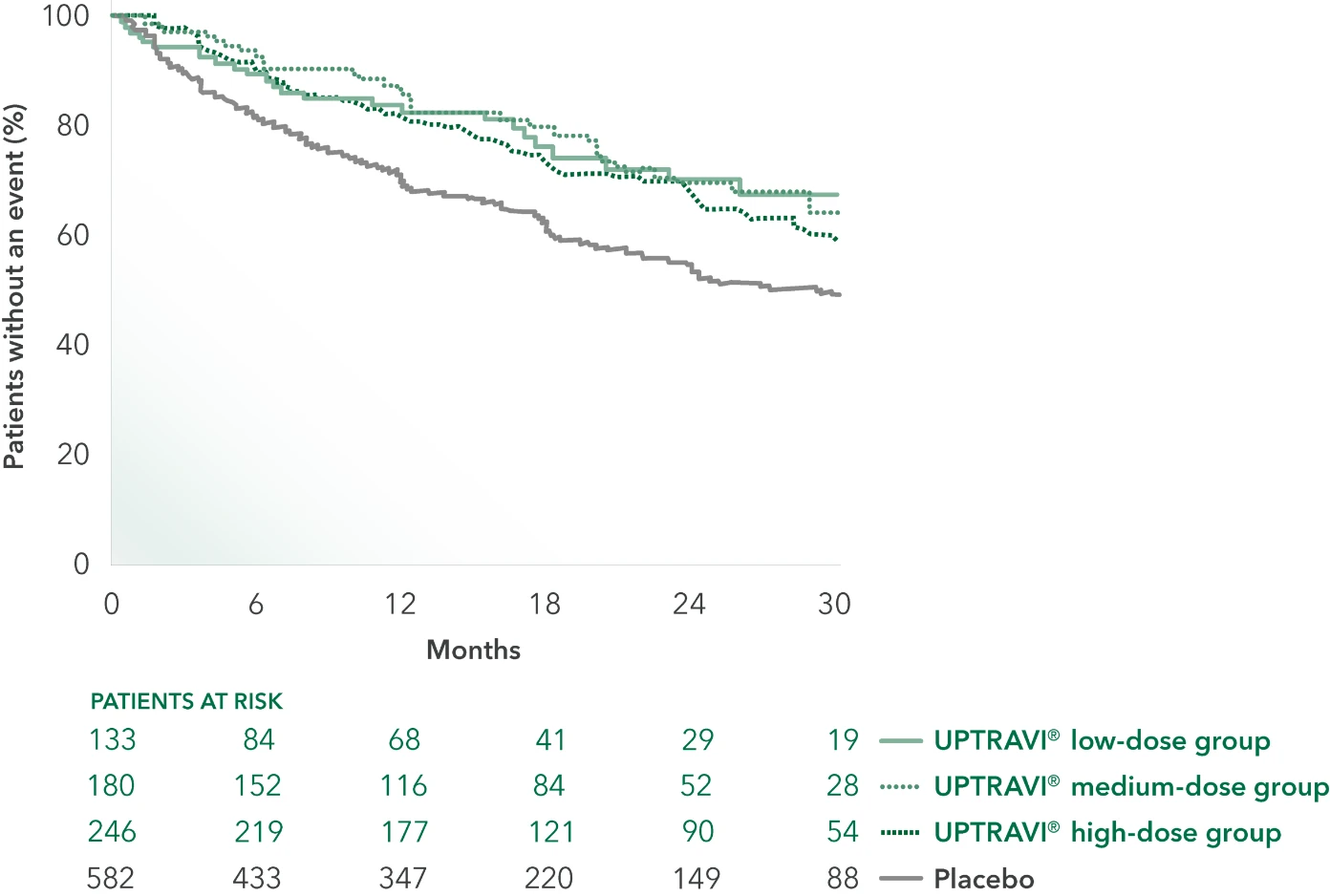
Efficacy by dose group of UPTRAVI® vs placebo3†:
- 40% RISK
REDUCTION(low dose:
200 mcg to 400 mcg)
HR 0.60 (95% CI: 0.41, 0.88) - 47% RISK
REDUCTION(medium dose:
600 mcg to 1000 mcg)
HR 0.53 (95% CI: 0.38, 0.72) - 36% RISK
REDUCTION(high dose:
1200 mcg to 1600 mcg)
HR 0.64 (95% CI: 0.49, 0.82)
UPTRAVI® doses were achieved across the 3 prespecified groups in the GRIPHON trial‡
- 200 mcg to 400 mcg BID (low dose): 23% of patients (n=133)
- 600 mcg to 1000 mcg BID (medium dose): 31% of patients (n=179)
- 1200 mcg to 1600 mcg BID (high dose): 43% of patients (n=246)
ADMINISTRATION
- Twice-daily dosing1
- No needles, pumps, or inhalers1§