Adverse reactions occurring more frequently (≥3%) with UPTRAVI® compared with placebo in the GRIPHON trial1
| ADVERSE REACTION | UPTRAVI® n=575 | Placebo n=577 |
|---|---|---|
| Headache | 65% | 32% |
| Diarrhea | 42% | 18% |
| Jaw pain | 26% | 6% |
| Nausea | 33% | 18% |
| Myalgia | 16% | 6% |
| Vomiting | 18% | 9% |
| Pain in extremity | 17% | 8% |
| Flushing | 12% | 5% |
| Arthralgia | 11% | 8% |
| Anemia | 8% | 5% |
| Decreased appetite | 6% | 3% |
| Rash | 11% | 8% |
Adverse reactions occurring more frequently (≥3%) with UPTRAVI® compared with placebo in the GRIPHON trial1
| ADVERSE REACTION | UPTRAVI® n=575 | Placebo n=577 |
|---|---|---|
| Headache | 65% | 32% |
| Diarrhea | 42% | 18% |
| Jaw pain | 26% | 6% |
| Nausea | 33% | 18% |
| Myalgia | 16% | 6% |
| Vomiting | 18% | 9% |
| ADVERSE REACTION | UPTRAVI® n=575 | Placebo n=577 |
|---|---|---|
| Pain in extremity | 17% | 8% |
| Flushing | 12% | 5% |
| Arthralgia | 11% | 8% |
| Anemia | 8% | 5% |
| Decreased appetite | 6% | 3% |
| Rash | 11% | 8% |
Other AEs and laboratory findings of interest3*
| ADVERSE REACTION | UPTRAVI® n=575 | Placebo n=577 |
|---|---|---|
| Hyperthyroidism | 1% | 0% |
| Hypotension | 5% | 3% |
| Syncope | 6% | 9% |
| Major bleeding event†‡ | 2% | 2% |
| Hemoglobin 8 g/dL§ | 1% | 1% |

Pivotal trial overall population: Adverse reactions during the dose adjustment phase1,3
Adverse reactions were more frequent during the titration phase. Once patients reached the maintenance phase, adverse reactions were less frequent.
No increased risk of bleeding with UPTRAVI® when administered with oral anticoagulants or platelet inhibitors.4
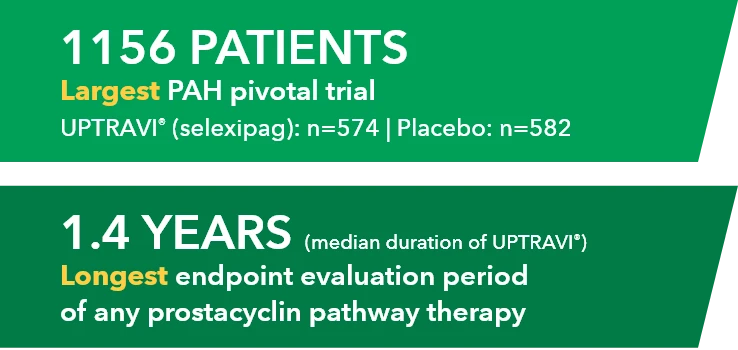
UPTRAVI®: 10 years of patient impact
VIEW LONG-TERM DATA